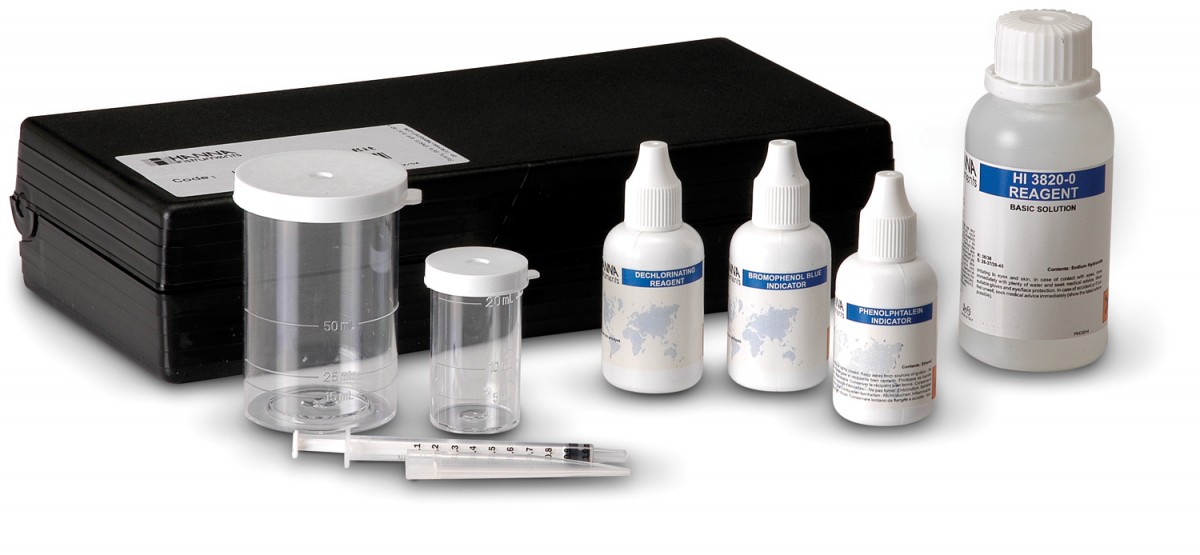
HI3820 CTK Acidity
฿2,782.00
Chemical test kit for the determination of acidity in the range 0 to 100 mg / l (ppm).
No. of Tests: 110 tests
0 in stock
With the use of diluted sodium hydroxide as the titrant and bromphenol blue or phenolphthalein indicators, the contribution of strong or organic acids can be determined. The measurement of the strong acid contribution to the sample acidity is known as methyl orange acidity. This is carried out by titrating with sodium hydroxide until the solution turns from yellow to green/blue (pH endpoint about 4.5). The total acidity caused by both mineral and organic acids is determined by titrating to an endpoint pH of 8.3, using phenolphthalein as an indicator. This is known as phenolphthalein inacidity.
Order Information:
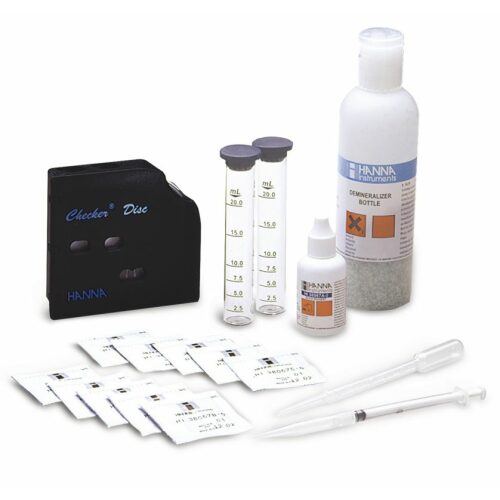
HI 3820 test kit comes with 10 mL dechlorinating reagent, bromophenol blue indicator, phenolpthalein indicator, acidity titrant, 10 mL calibrated vessel, 50 mL calibrated vessel, and calibrated syringe.
![]() Manual: Download
Manual: Download
| Method | Titration | |
| Range | 0-100 mg/L 0-500 mg/L |
|
| Smallest Increment | 1 mg/L 5 mg/L |
|
| Chemical Method | Methyl-orange/Phenolphthalein | |
| Number of Tests | approx. 110 | |
| Weight | 910 g |
Related products
All Product
Chemical test kit for the determination of hydrogen peroxide in the range 0.00 to 2.00 mg / L / 0.0 to 10.0 mg / l (ppm). 100 Tests
All Product
A test kit for the determination of chemical copper in the range 0 to 2.5 mg / l (ppm). 100 Tests
All Product
Chemischer Testkit zur Bestimmung der Härte im Bereich 40 bis 500 mg/l (ppm). Packungsgröse: 50 Tests
All Product
Chemical test kit for the determination of chloride in the range 0 to 100 mg / l / 0 to 1000 mg / l (ppm).
All Product
A test kit for the determination of ammonium chemical seawater in the range 0 to 2.5 mg / l (ppm).
All Product
The HANNA test kit for ozone determines the ozone concentration in water via checker disc. The reaction between ozone and the reagent causes a pink tint in the sample which is proportional to the ozone concentration.
All Product
Chemical test kit for the determination of free and total chlorine in the range 0.0-0.70/0.0-3.5 mg / l (ppm). 200 tests
All Product
Test kit for the chemical determination of silicate in the range 0-1 mg / l (ppm). 100 Tests